During chemical reactions, energy is either released to the environment (exothermic reaction) or absorbed from the environment (endothermic reaction). During chemical reactions, bonds are broken in the reactants and new ones are made in the products. Bond-breaking is an endothermic process and bond-making is an exothermic process. The average bond dissociation energies of some chemical bonds are shown in the following table.
|
Selected Bond Energies |
|||
| Bond | Bond Energy(kJ/mole) | ||
| H-H | 432 | C=O | 799 |
| O=O | 494 | C-C | 347 |
| O-H | 460 | C=C | 611 |
| C-H | 410 | C=C (aromatic) | 519 |
| C-O | 360 | N=O | 623 |
For any chemical reaction, the overall energy change, the enthalpy of reaction(DH), is the difference of all the energy absorbed in bond-breaking and all the energy released in bond-making.
H = S BE(bonds broken)- SBE(bonds formed)
The negative value of the Enthalpy of Reaction indicates that the reaction is a heat producing or exothermic.
The energy produced in exothermic reactions can be used directly or be converted to other energy forms.
Redox Reactions
An oxidation-reduction or redox reaction is one in which electrons are transferred between two species. The rusting of iron is an example of a redox reaction.
In this reaction iron metal losses electrons (is oxidized) and the oxygen gains electrons (is reduced).
In a redox reduction something is always oxidized while another species is reduced.
Oxidation is easy to recognize when an element changes changes oxidation state to become an ion. However when dealing with organic molecules, atoms are joined by covalent bonds rather than ionic ones so oxidation is not as easily recognized.
In organic molecules, an atom is oxidized if during a reaction it becomes bonded to a more electronegative element which pulls electron density away from it.
In general, oxidation occurs if the oxygen content of a covalently bonded molecule increases or if the hydrogen content decreases.The carbon atom in methane (CH4) is more reduced than the carbon atom in carbon dioxide (CO2)
Combustion of Fossil Fuels
Combustion is a reaction with oxygen. In the case of the combustion of fossil fuels, the combustion reaction is what we think of as a burning process. In the combustion reaction, the species reacting with the oxgyen is oxidized (because oxygen is very electronegative). Fossil fuels are composed primarily of hydrocarbons (molecules containing primarily carbon hydrogen bonds). In these molecules carbon is in a very reduced state. During the combustion reaction, the hydrocarbon molecules are converted to carbon dioxide and water.
Every mole of methane (16 g) releases 810 KJ of energy on burning.
Combustion energetics can be estimated from the bond energies for all the classifications of fossil fuels. The amount of energy released is dependent on the oxidation state of the carbons in the hydrocarbon which is related to the hydrogen/carbon ratio. The more hydrogen per carbon, the lower the oxidation state and the more energy that will be released during the oxidation reaction. Thus the greater the H/C ratio, the more energy release on combustion.
RELATED VIDEO



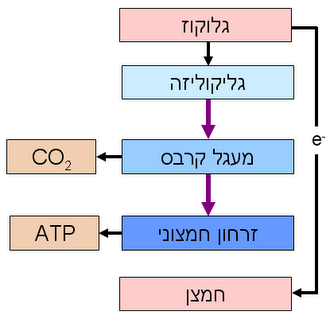 Cellular respiration is the set of the metabolic reactions and processes that take place in the cells of organisms to convert biochemical energy from nutrients into adenosine triphosphate (ATP), and then release waste products. The reactions involved in respiration...
Cellular respiration is the set of the metabolic reactions and processes that take place in the cells of organisms to convert biochemical energy from nutrients into adenosine triphosphate (ATP), and then release waste products. The reactions involved in respiration...
 In physics, energy (Ancient Greek: ἐνέργεια energeia "activity, operation") is an indirectly observed quantity. It is often understood as the ability a physical system has to do work on other physical systems. Since work is defined as a force acting through a...
In physics, energy (Ancient Greek: ἐνέργεια energeia "activity, operation") is an indirectly observed quantity. It is often understood as the ability a physical system has to do work on other physical systems. Since work is defined as a force acting through a...








