FUEL 100%
PUSHING THE PISTONS 35%
OVERCOMING ENGINE FRICTION AND PUMPING THE AIR AND FUEL
(typical US driving condition) 20%
Are we stuck with ~20% auto engine efficiency?
What can be done?
- Run the engine fuel-lean, that is, use excess air. It is well known that fuel-lean running improves the efficiency. In the old days, under cruising conditions, the engines always ran lean – about 15% excess air - this was economical. So what happen to change this? The problem is the three-way (CO, UHC, NOx) catalyst used on engine exhausts. This only works if the engine air/fuel ratio (by mass) is stoichiometric (chemically correct). For gasoline this ratio is 14.6:1. The engine computer, acting in concert with the engine air flow sensor, electronic fuel injectors, and exhaust oxygen sensor, maintains the stoichiometric ratio for most of your driving. Only at this ratio can the catalyst both oxidize the CO and UHC (to CO2 and H2O) and chemically reduce the NOx (to N2). (UHC = unburned hydrocarbons.) What humankind needs is a lean-NOx catalyst. Then we could have increased efficiency and continue to be clean!
- Also needed are ways to improve lean flammability in gasoline engines. That is, the ability to burn real lean is limited by the fuel. If the gasoline-air mixture is too lean, the flame will not have enough speed to get across the cylinder in the time permitted by the engine RPM the driver wants, or the flame will not even start – the cylinder misfires, and then the catalyst has to oxidize a huge amount of UHC and thus may overheat (which might mean you have to buy a new catalyst).
Background:
A first course on thermodynamics may teach the efficiency of the Otto cycle (which is the ideal cycle used to simulate the gasoline spark ignition auto engine). Such a course would derive the following equation for the Otto cycle efficiency:
= 1 – 1/rvg-1The compression ratio of the engine is rv. Actually, this is a volume ratio. It is the ratio of the volume in a cylinder when the piston is at the bottom of the cylinder to the volume in the cylinder when the piston is at its top position: rv = Vbottom/Vtop.
Most auto engines have compression ratios in the 9 to 10.5 range. We note: the higher the compression ratio, the higher the efficiency! The g parameter is the ratio of the specific heats, ie, the constant pressure specific heat over the constant volume specific heat. In practical terms, the higher the g, the higher the efficiency. A gas such as helium or argon, composed only of atoms, has the highest g possible, 1.67. Room air on the other hand, being mainly composed of O2 and N2 molecules has a g of 1.4. Fuel vapor has g less than that of air. The mixture of air and gasoline vapor inducted into the engine has a g of about 1.35. As this mixture is compressed and heated during the compression stroke, its g drops to about 1.33. Upon combustion (when the piston is near its top position), the fuel is oxidized to CO2 (and some CO) and H2O, and g drops further. It drops into the 1.20-1.25 range. The overall, effective g for the whole cycle for use in the efficiency equation above is about 1.27.
The rule of thumb is: the greater the complexity of the molecules, the lower the g. The lower limit is 1. Argon and helium atoms only translate, that is, they move along straight paths until they encounter another atom. Room air molecules translate and rotate (about 2 of their axes). Hot air starts to vibrate (as two nuclei connected by a spring). Molecules of fuel vapor have a lot of opportunity to vibrate, even at room temperature. The products of combustion vibrate. However, only the translation of the molecules PUSHES the piston. The other modes of molecular motion do nothing for pushing the piston. Thus, as g drops (indicating more vibration of the molecules), h drops. A lean engine (ie, an engine with excess air) has a cooler combustion process and more air relative to fuel than the typical engine with a chemically correct mixture. Thus, its g is higher, and its h is greater.
Plug g = 1.27 into the efficiency equation above, assume rv = 10, and you get h = 0.46. Multiply this by about 0.75 to account for real cycle effects (such as the time it takes to burn, heat losses to the coolant, and exhaust valves that open before the piston fully reaches bottom position) and you have h = 0.35. This is the efficiency (given above) of using the chemical energy of the fuel to push the pistons. Multiply this by the mechanical efficiency of the engine, which accounts for the mechanical friction in the engine and for the air (and fuel) pumping work that has to be done, and you have the final, or overall efficiency of the engine. Of course the mechanical efficiency varies with driving conditions. The higher the RPM of the engine, the greater the friction loss. The more closed the throttle (ie, the farther your foot is off the pedal), the higher the pumping loss. For typical US driving, the resultant overall efficiency of the engine is about 20%. Note, your pedal is not really a gas pedal, it is an air pedal! Add the tranny and real axle mechanical friction losses (or the transaxle friction losses) and the drain of a few essential accessories, and you arrive at a 15% fuel-to-wheel efficiency for the typical auto driven in the US.
However, the diesel engine is not subject to this limitation. It runs at high compression ratio. In part, this explains its high efficiency. It also runs lean, and its pumping work is low, further increasing its efficiency over the gasoline engine. Humankind needs quiet, smoke-free, odor-free diesels!
- Higher compression ratio. Here, we are limited by autoignition of the gasoline – knock. That is, if the gasoline engine compression is above about 10.5, unless the octane number of the fuel is high, knocking combustion occurs. This is annoying and if persistent, damage to the engine can occur. Thus, gasoline engines are limited in their efficiency by the inability of the fuel to smoothly burn in high compression ratio engines.
- We need new cycles put into...
RELATED VIDEO



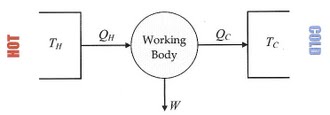 In thermodynamics, a heat engine is a system that performs the conversion of heat or thermal energy to mechanical work. It does this by bringing a working substance from a high temperature state to a lower temperature state. A heat "source" generates thermal energy...
In thermodynamics, a heat engine is a system that performs the conversion of heat or thermal energy to mechanical work. It does this by bringing a working substance from a high temperature state to a lower temperature state. A heat "source" generates thermal energy...








